Radiotherapy services in Abu Dhabi
Overview
The specialty of Radiation Oncology at Mediclinic in Abu Dhabi provides highly specialised, individualised and comprehensive radiotherapy care to patients with cancers and also treatment for some benign conditions. We have a highly experienced team of Radiotherapy staff who are committed to providing treatment in an individualised, evidence based, state of the art and most importantly in a patient centric manner.
Through our comprehensive cancer care approach, we provide a truly multidisciplinary management of cancer through the involvement of various experts such as Surgical Oncologists, Medical Oncologists, Radiation Oncologists, Radiologists and Pathologists in each patients care, through the Tumour board.
Radiotherapy is the treatment of cancers using ionising radiation to destroy or control cancer cells. It is estimated that Radiotherapy cures around 40% certain types of cancer, either on its own or in conjunction with surgery and/or chemotherapy.
Where cure is not possible, it can be effectively used to control cancer or alleviate symptoms such as pain, bleeding, etc. It is an integral part of the cancer treatment in 1 out of 2 patients diagnosed with cancer, at some point during their cancer journey.
It is also used to treat some benign (non-cancerous) conditions that cannot be controlled by other methods, such as surgery.
The course of radiation at Mediclinic in Abu Dhabi is prescribed on an individual basis, dependent on size, type and location of the problem, as well as the individual’s general health. Each patient’s treatment is planned individually and delivered by a team of highly qualified experts in the field including Radiation Oncologists, Physicists, Radiation Therapists and Dosimetrists.
How is radiation therapy delivered?
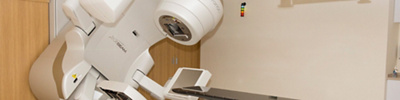
Mediclinic in Abu Dhabi uses the latest advanced radiation therapy techniques and technology to deliver accurate and safe radiation to tumours. We use a state of the art Varian Truebeam linear accelerator with on board imaging for image guidance and respiratory gating for complex treatments such as DIBH and SBRT.
The treatment is usually delivered each day (Sunday to Thursday) over a variable number of weeks. This allows the radiation to target cancer cells and provides healthy cells an opportunity to recover, over the weekend.
External beam radiation does not make you radioactive therefore it is safe for patients to be around other people, including children and pregnant women.
For any enquiries please call us on 0559181198 or email us at MAIRRadiotherapy@mediclinic.ae
Our Machines
Linear Accelerator
At Mediclinic Airport Road, we have the latest Varian Linear Accelerator – TrueBeam®.
This is a high precision radiotherapy system which integrates into one machine, precision engineered hardware with advanced software to accurately plan, shape the radiation beam into any target shape and deliver it with submillimeter accuracy, RapidArc® treatment deliver system for faster delivery of daily treatments and on-board imaging, including cone beam CT, for accurate targeting of the cancer and sparing of the normal tissues.
Armed with these technologies we are in a position to gain better tumour control with fewer side effects.
The ‘Real time tumour tracking’ capability, allows to accurately target moving tumours such as those in the lung and to deliver the Deep Inspiratory Breath Hold (DIBH) technique, the standard of care in treating breast cancer.
With the Varian Truebeam®, we can deliver a variety of techniques treating all types of cancer including stereotactic radiosurgery (SRS) with submillimeter accuracy to treat your cancer.
CT Simulator
Prior to any radiotherapy treatment, a highly specialized radiotherapy CT simulation scan needs to be acquired.
The Siemens Somatom go.Sim allows us to obtain high quality images with precision of position to accurately plan and deliver your radiation treatments.
With the help of this cutting edge equipment we have the ability to monitor the patient’s breathing cycle where necessary and generate 4DCT scan capturing tumour movement through the entire breathing cycle.
This state of the art CT scanner allows us to raise the bar when it comes to treatment precision and minimizing side effects.
Treatments offered
Radiotherapy treatments may be delivered in several ways, using different types of radiation, with external beam radiotherapy using photons being the commonest method. With the advance of technology, various techniques are used to precisely target the area of concern, while sparing the normal surrounding structures.
We use various state of the art techniques to accurately target the tumour while sparing the surrounding normal tissues in order to maximise the treatment benefit while minimising the side effects Learn more about your treatment pathway.
The various techniques we use for treatments are
- 3DCRT
- IMRT
- VMAT/RapidArc
- IGRT (image guided radiotherapy)
- DIBH (Deep inspiratory breath hold)
- Stereotactic body radiotherapy (SABR/SBRT)
- SRS
Cancer types treated
We offer treatment to various types of cancers as listed below
- Breast
- Rectum
- Prostate
- Gynaecological cancers
- Pancreas
- Bladder
- Anal cancer
- Lung
- Thymic tumours
- Head and neck cancers
- Salivary gland cancers
- Brain tumours
- Lymphoma
- Sarcoma
- Skin cancers (melanoma, squamous cell cancer, basal cell cancer)
- Palliative radiotherapy to various sites
- Some benign conditions such as Keloids, Hypertrophic bone formation, Thyroid eye disease
Follow up care
During and after radiotherapy, you can be assured of professional support and ongoing care from your Radiation Oncologist, Nurse and Radiation Therapist for guidance and support in managing side effects.
After completion of your treatment, our team will arrange your follow up with the most relevant care provider to follow up and assess tumour response to treatment, manage long term effects or continue other treatments. This may be your Radiation Oncologist, Medical Oncologist, Surgeon or Physician.
If you experience any side effects during or in the 4 weeks after your Radiation therapy treatment, please contact our specialist nurse for advice.
For Doctors
Actinic keratosis (AK) and cutaneous Bowen’s disease (SCC in situ)
Background
AK (solar keratosis) is a precancerous skin condition caused by long-term exposure to ultraviolet light. Up to 5% of AK on skin may become invasive skin cancers. Bowen’s disease is a form of SCC in situ that can be transformed into invasive cutaneous SCC. Bowen’s disease is more common on the lower leg where healing after RT can be impaired.
Management
AK and Bowen’s disease are generally treated by dermatology or surgical services. Patients with persistent or recurrent AK or skin Bowen’s disease may benefit from referral to the radiotherapy team.
Radiotherapy
Radiotherapy should be considered to treat symptomatic Bowen’s disease of the skin that is refractory or recurrent after other treatment modalities, taking into account the site of disease and likelihood of healing after treatment.
Doses from 25–70 Gy would appear to be effective and local recurrence rates are equally low in patients treated with high- and low-dose radiotherapy regimes. An Australian review suggested a dose fractionation schedule of 40–50 Gy in 10–20 fractions using superficial (110–150 kV) energy photons will achieve a local control rate of 95–100%.
Radiotherapy in AK has been used mainly in salvage setting. Most evidence indicates prolonged duration of control in heavily pretreated patients.
Brachytherapy (high-dose rate) should be considered in in convex shapes such as on the scalp or dorsum of hand or foot skin, while large areas of field-change may benefit from newer EBRT techniques such as VMAT
Dupuytren’s disease of the hand
Background
Dupuytren’s disease is a common benign proliferative disorder of the palmar fascia and is part of a group of fibromatoses that includes plantar fibromatosis (Ledderhose disease) and penile fibromatosis (Peyronie’s disease). Dupuytren’s disease tends to present in the sixth and seventh decade of life but can present earlier or later. The cause of these fibromatoses is unknown, but they appear to have a genetic component. Additional risk factors include prior hand trauma, epilepsy and diabetes mellitus.
The early stage consists of subcutaneous palmar nodules, skin retraction and cord formation. The disease course is variable, but is more severe in males, those with a positive family history, early onset, bilateral disease and where there are ectopic lesions (such as Peyronie’s disease). Eventually the cords thicken and contract and cause fixed flexion of the metacarpophalangeal or proximal interphalangeal joints of the fingers, known as Dupuytren’s contracture. There is no cure for Dupuytren’s disease, and it is most often treated in the advanced stages, where there is significant (for example >30 degrees) contracture, particularly where hand function is impaired.
Surgical Management
Surgical management is directed towards releasing the contracture and improving function. There are two main surgical methods for release of contractures: Fasciectomy (Limited or radical) and needle aponeurotomy.
Radiotherapy
Radiotherapy is effective in the early stages of Dupuytren’s disease, where there is no contracture or a contracture of up to ten degrees. Patients with more advanced disease should not be treated with radiotherapy but may be offered surgical release. Due to the variable progression of this disease, only patients whose disease has progressed within the last 6–12 months should be treated.
The aim is to treat nodules and cords to the periosteum of the hand bones, for a depth of 5–15 mm. Therefore, 120–150 kV photons or up to 6 MeV electrons with appropriate bolus would be reasonable. Proximal and distal margins of 1–2 cm on palpable nodules and cords, with 0.5–1 cm lateral margins should be used.
RT dose: the regimen of choice is 30 Gy in ten fractions, consisting of two phases of 15 Gy in five fractions with a gap of 6–12 weeks between the two phases. An alternative fractionation is 21 Gy in seven fractions on alternate days over two weeks.
Long-term side effects of radiotherapy are anhidrosis, skin atrophy and reduced wound healing. The risk of radiotherapy induced cancer is estimated to be about 0.02% higher than the probability of dying from cancer without RT (estimated to be ~24 ± 0.26%). Since the excess risk is very small compared with the background risk it is impossible to evaluate this accurately in a clinical study.
Graves’ Orbitopathy (thyroid eye disease)
Background
Grave’s Orbitopathy (GO) is a rare autoimmune condition affecting 3.3 –16 women and 0.9 –2.9 men per 100,000 people annually. 85% of patients have thyrotoxicosis within 18 months of diagnosis but Orbitopathy can precede thyroid dysfunction. The extraocular muscles and retro-ocular connective tissues are infiltrated by lymphocytes leading to oedema. Similar changes can occur in the eyelids and anterior orbital tissues. In most people, both eyes are affected. In the presence of visual disturbance it is important to exclude optic nerve compression when blurring will not improve with blinking or refraction.
Management
Control of thyroid dysfunction, stopping smoking and selenium supplementation are recommended in all patients. Topical treatments like artificial tears and lubricants can be helpful. First-line therapy is intravenous (IV) Methylprednisolone with or without mycophenolate and is effective in 50–80% of patients. If there is no response then various second-line options exist – repeating IV steroids, oral steroids with Cyclosporine or Azathioprine, orbital RT with steroids or immune-modulating drugs such as Rituximab, Teprotumumab or Tocilizumab.
Radiotherapy
Ensure all patients being considered for orbital radiotherapy should have been assessed in a thyroid eye clinic with ophthalmologist and endocrinologist input.
Orbital radiotherapy should be considered in moderate-to-severe active GO that has not responded to IV Methylprednisolone. The 2021 EUGOGO guidelines only recommend RT as second-line treatment for moderate-to-severe active disease after IV methylprednisolone has not been effective.
Radiotherapy is usually combined with oral or IV steroids to improve effectiveness and reduce side-effects. The recommended dose is usually 20 Gy in ten daily fractions over two weeks, though lower doses may also be effective.
Orbital radiotherapy should be avoided in people who have diabetic retinopathy or uncontrolled hypertension. Any diabetes and younger age are relative contraindications.
Orbital radiotherapy is usually well tolerated. Transient exacerbation of eye symptoms appears to be minimised by the concurrent use of steroids. Radiotherapy may increase the risk of a cataract. The risk of radiotherapy-induced cancer is estimated to be about 0.2%.
Head and neck Paraganglioma
Background
Paragangliomas (PG) are rare vascular tumours arising from neuroendocrine cells in the paraganglia. Carotid body tumours typically present with a mobile slow-growing neck mass and can be associated with cranial nerve palsies (X, XII). Jugular PGs originate at the jugular bulb at the skull base and may be associated with bone destruction, often presenting with cranial nerve palsies (IX–XII). Tympanic PGs usually originate within the middle ear and present with associated ear symptoms. Vagal PGs can present as an intraoral parapharyngeal mass and can cause defects in cranial nerves X–XII. PGs are usually benign hypervascular lesions. Malignancy cannot be predicted histologically, and is defined by the presence of regional or distant metastases.
Management
Most head and neck PGs demonstrate an indolent growth pattern and therefore the aim of treatment of PGs is to minimise or reduce morbidity rather than to improve survival. Options for treatment include active surveillance, surgery or radiotherapy. If immediate treatment is not required, observation is often adopted to determine tumour behaviour. If treatment is required, the choice depends on tumour site, extent of the lesion, presence of synchronous tumours, mutation status, co-morbidity and potential for treatment-related morbidity. Local control rates are high following treatment, although surgical morbidity can be significant and late morbidity from radiotherapy needs to be considered. The aims of treatment are different with surgery aiming to achieve a complete resection while radiotherapy aims to prevent disease progression.
Radiotherapy
Radiotherapy is preferred for inoperable lesions and more advanced lesions due to the morbidity of surgery.
A radiotherapy dose of 45–54 Gy in 18–2 Gy per fraction is recommended. For stereotactic radiosurgery (SRS) a typical marginal prescription dose is of 12–15 Gy as a single fraction.
The long-term risks of radiation exposure are primarily related to radiation-induced cancers to the brain so the dose to the brain should be minimised. In some cases there may also be sensorineural hearing loss.
Heterotopic ossification of the hip (HO)
Background
HO is the abnormal formation of mature bone within extra skeletal soft tissues. It occurs most commonly after trauma or surgical procedures, for example after total hip arthroplasty. The origin of the new bone is not entirely clear, but it is thought to result from the inappropriate differentiation of pluripotential mesenchymal cells into osteoblastic stem cells. Under the influence of inductive agents (bone morphogenic proteins), these cells form new bone. HO can occur at any age, although most hip replacements occur between the ages of 50–80 years. In many patients HO is asymptomatic, but in some patients the new bone may cause symptoms such as swelling and tenderness, pain and limited range of motion. Risk factors include prior HO, trauma and muscle injury, and disorders such as Paget’s disease and ankylosing spondylitis.
Surgery
Symptomatic HO is treated with surgery, which is delayed until at least six months after the traumatic episode to allow the bone to mature and for the inflammation to settle. Preventative measures, either NSAIDs or radiotherapy, may be used to minimise the risk of recurrence or to reduce the initial occurrence rate in high-risk situations.
Radiotherapy
Radiotherapy and NSAIDs are both effective in the prevention of HO but NSAIDs are more cost effective. Radiotherapy should be considered in people who are unable to take NSAIDs or who are at risk of more severe HO. It should be avoided in younger patients (for example <50 years). However, given the low dose recommended, if there are contraindications or lack of response to NSAIDs, radiotherapy could be considered for younger patients, with appropriate counselling regarding the risk of radiation-induced malignancy and infertility.
RT can be given either pre- or postoperatively and should be delivered within four hours before surgery or within 96 hours after surgery. A single fraction of 7 Gy of Radiotherapy seems optimal and is equivalent in efficacy to increased doses and fractions, with a likely reduction in the risk of second malignancy. The discussion above covers the prevention of HO of the hip. RT has been used to prevent HO at other sites, but data on its success are more limited.
Juvenile nasopharyngeal angiofibromas
Background
Juvenile nasopharyngeal angiofibromas (JNA) are benign rare vascular tumours. They are most common in adolescent boys/young men between the ages of 9–19 years old. JNAs are thought to arise from the superior margin of the sphenopalatine foramen at the posterolateral wall of the roof of the nasal cavity. Presenting symptoms are most commonly unilateral nasal obstruction and recurrent unprovoked profuse unilateral epistaxis. Other reported symptoms include nasal discharge, cheek swelling, proptosis, and anosmia, headaches and hearing impairment. A pink or bluish nodular mass is typically seen in the roof of the nasopharynx. MRI with gadolinium is the diagnostic imaging investigation of choice. CT can provide complementary anatomical information. Biopsy is not usually required and carries a high risk of bleeding. Although considered benign neoplasms, JNAs can demonstrate locally aggressive behavior infiltrating adjacent structures, with a tendency to spread through the foramina in the base of skull into the cranium, leading to significant morbidity.
Management
Surgery is regarded as the treatment of choice for JNA and should aim for clear margins, as inadequate margins are associated with significant failure rates. Potential postoperative morbidity includes disturbance of mid-facial growth following craniofacial resection.
Radiotherapy
Primary radiotherapy is an effective treatment modality if the disease is deemed incompletely resectable without excess morbidity, and in patients who are unsuitable for surgical treatment.
Surgery or radiotherapy can be considered for recurrent disease.
Conventionally fractionated doses in the mid-range of 35–45 Gy are recommended, with a dose of 36 Gy in 20 daily fractions being appropriate, with no evidence of a dose response with doses in the higher end of this range.
It is appropriate to consider proton beam therapy to minimise the risk of late treatment-related side-effects due to the high conformity of treatment.
The major concern with the use of RT for these young patients is late toxicity. Only a few cases of second malignancies have been described. Cataract has been reported more commonly. Other potential late side-effects include hypopituitarism and xerostomia.
Keloid scarring
Background
Keloid scars are classified as benign dermal fibro-proliferative growths and represent abnormal healing responses to injury. They occur in 1–16% of wound healing with the highest incidence in black skin. Keloids result in raised scars that may be red or hypo- or hyper pigmented. They are often cosmetically disfiguring but can also cause itching and pain. In contrast to hypertrophic scars that are limited to the damaged skin, keloids extend outside the confines of the original wound and do not spontaneously regress. Keloids occur on the upper chest, shoulder/scapula regions and earlobes. They are most common between the ages of 10–30 years.
Management:
Corticosteroids are often used as a primary and secondary treatment (such as after surgery) for keloids and have been shown to inhibit the formation of collagen by fibroblasts. Triamcinolone is the steroid most often used as a first- or second line treatment. When surgery is used as the sole modality, the reported recurrence rates range from 45–100%. It is therefore generally used only as part of multimodal therapy. Other techniques: Silicone gel sheet application or compression with bandages, intralesional interferon, cryotherapy, bleomycin, ultraviolet irradiation, topical imiquimod, photodynamic therapy, electrical stimulation and laser therapy are not widely used in clinical practice.
Radiotherapy
It is postulated that radiotherapy effectively prevents or treats keloids by suppressing angiogenesis, inhibiting immune cell function and inhibiting histamine release from mast cells, which in turn inhibits the proliferation of fibroblasts.
Radiotherapy as a sole treatment should be considered in older or frail patients, especially when symptomatic or in those with huge keloids. A study noted that radiotherapy can immediately reduce pain and itchiness while reduction in size and color of the keloids may take months.
If adjuvant radiotherapy is to be used, it should ideally be administered less than 24 hours after surgery. It should not be used more than 72 hours after surgery.
Superficial or orthovoltage (generally 60–120 kV) electrons or brachytherapy can be used. There is no one agreed schedule that can be recommended and fractionation varies among centers, therefore the use of postoperative RT should follow local protocols and expertise. Examples of common fractionations
For high-recurrence sites (anterior chest wall, scapular region and suprapubic region) 18 Gy in three fractions over three days.
For earlobes 8 Gy in one fraction.
Other body sites, including auricle (but not earlobe) 15 Gy in two fractions over two days.
The most commonly seen late side-effects are telangiectasia or depigmentation in up to 19% of cases. The risk of secondary malignancy is approximately 0.02% for a field size of 60 cm2 at a dose of 30 Gy in an individual of 45 years at the time of treatment.
Lentigo maligna
Background
Lentigo maligna (LM) is a slow-growing melanoma in situ occurring in chronically photo-damaged skin, predominantly in older people. It is a macular, irregularly hyperpigmented skin lesion presenting on the sun-exposed head and neck region, typically on the cheeks, nose, forehead and ears. If untreated, LM has the potential to become invasive and progress into lentigo maligna melanoma (LMM). Estimations on the risk of development of LMM from LM vary from a 5–20% lifetime risk of general progression to a 50% risk reported following excision of LM with incomplete margins. Biopsy is the gold standard of LM/LMM diagnosis, but the preferred excisional biopsy may not be practical due to the larger size of these lesions and where they are located near critical structures such as the eyelid. Incisional biopsy risks sampling error due to the area within the lesion selected for testing.
Management
Management is challenging due to high rates of recurrence, with optimal results following complete surgical excision compared with non-surgical techniques. Margin-guided techniques such as Mohs micrographic surgery, staged excision or the spaghetti technique produce the best results with recurrence rates of <5%. However, surgery may be contraindicated depending on the size and location of the lesion, the resulting cosmetic and functional outcomes and the co-morbidities of the patient. Imiquimod is only suggested where neither surgery nor radiotherapy are an option. Factors to consider in choice of treatment include the size and location of the lesion, patient age, co-morbidity and patients’ preference.
Radiotherapy
In contrast with surgery, Radiotherapy has the advantage of being able to treat large lesions with wide margins. Radiotherapy can be also used adjuvantly following incomplete surgery with margin involvement.
Treatment can be delivered as external beam or brachytherapy. The use of Grenz ray, superficial, orthovoltage and electron therapy have been reported. Evidence to guide optimum doses is very limited, although doses similar to those used in the treatment of skin cancer are appropriate and are tailored to the site and size of the lesion and likely cosmesis. Examples are 54 Gy in 27 fractions (definitive), 50 Gy in 25 fractions (adjuvant). Alternative schedules include 40–45 Gy in 10 fractions over two weeks and 50 Gy in 15 fractions over three weeks.
Histological evidence has shown that LM can extend beyond clinically visible abnormality. Therefore, treatment doses should be delivered to encompass at least a 1cm around the clinically detectable lesion and to 5mm depth.
The risk of a secondary malignant skin cancer is estimated at about 0.017% for an individual receiving 50 Gy to the skin at age 60. More important is the potential for the affected area, and the margin around it, to develop a subsequent malignant melanoma resulting from inadequate control of the original disease; consequently, careful long-term monitoring of the skin is important. Other late toxicity includes alterations of pigmentation, telangiectasia, alopecia and skin atrophy.
Pigmented villonodular synovitis (PVNS) / tenosynovial giant cell tumour (TSGCT)
Background
PVNS and giant cell tumours of tendon sheaths are rare proliferative processes involving synovial membranes and/or extra-articular tissues. These are now considered the same disease and termed Tenosynovial giant cell tumour (TSGCT), further sub-classified as nodular or diffuse.
The disease has a variable course and, while usually benign, may be destructive, resulting in major symptoms and loss of function leading to amputation. Optimum treatment is not always clear, and little information exists with respect to the role of RT in comparison with other modalities.
Management
The standard surgical approach is synovectomy, either as an open procedure or more recently via an arthroscopy procedure. High local control rates are achieved for patients with localised TSGCT with synovectomy but for diffuse disease local recurrence risk may be of the order of 20–50%.
More recently trials using tyrosine kinase inhibitors have shown worthwhile responses but questions over optimal treatment duration and control rates after stopping therapy remain.
Radiotherapy
Ionising radiation, either in the form of external beam radiotherapy or intra-arterial instillation of radionuclides, has been used for several decades, generally given postoperatively to reduce the risk of recurrence following synovectomy. Radiotherapy could be also used for the treatment of patients with severe symptoms and for those who may otherwise need to be considered for an amputation.
For patients with diffuse TSGCT, high local control rates for surgery and postoperative
RT are achieved with low toxicity. Typical RT doses are in the region of 35–40 Gy in
15–20 daily fractions.
Plantar fasciitis
Background
The plantar fascia is a band of fibrous tissue that runs along the plantar surface of the foot and extends from the calcaneus bone to the metatarso-phalangeal joints. Plantar fasciitis is a very common condition, which causes heel pain in approximately 10% of the population and is a combination of inflammation and degeneration of the plantar fascia. It is most common in people between the ages of 40–60 years. However, it can occur at any age. It is twice as common in women as it is in men and is also common in athletes. It is caused by mechanical overload, which may be due to a combination of obesity, prolonged standing and walking or intense exercise, and biomechanical disturbances of the foot or lower leg. In 80% of patients complete resolution is achieved in 12 months, but some patients have more prolonged and disabling symptoms.
Management
Plantar fasciitis is a clinical diagnosis, but an ultrasound scan may be useful to rule out other causes of heel pain. In most patients, simple conservative measures are all that is required, including resting, weight loss, analgesia, icing, stretching exercises, footwear changes and orthotics. For those cases where symptoms do not resolve with simple measures, various other treatments may be considered, including steroid injections, extracorporeal shockwave treatment (ESWT), ultrasonic tissue repair and surgery.
Radiotherapy
Radiotherapy is effective and may be considered for patients who have had plantar fasciitis for more than six months and who have failed conservative management.
Dose and technique: 3–6 Gy in six fractions (0.5–1 Gy per fraction) over three weeks delivered using a single lateral field, a parallel-opposed pair of lateral fields or 200–250 kV photons.
The risk of radiotherapy-induced cancer (RIC) after RT for plantar fasciitis is estimated to be 0.02%. This estimate is based on a field size of 60 cm2 but the risk increases or decreases with the field size. The risk decreases with increasing age at treatment. As a matter of course, patients should be counselled as to the risk of RIC, which should be more strongly emphasised in younger patients. Other possible consequences of radiation exposure is slight increase in pain during RT.
Plantar fibromatosis (Ledderhose disease)
Background
Ledderhose disease (plantar fibromatosis) is a rare benign hyperproliferative fibromatosis of the plantar fascia of the foot. It is histologically identical to Dupuytren’s disease of the hand, and the two conditions coexist in 20–30% of cases. The underlying cause is unclear, but there is an association with genetic factors, smoking, alcoholism, diabetes mellitus and anti-epileptic use. The symptoms usually start in the third or fourth decade but may rarely affect children and young adults. Plantar fibromatosis presents as nodules attached to the central and medial part of the plantar fascia, which may cause discomfort and difficulty with walking and fitting shoes. Contractures of the toes occur rarely.
Management
Non-invasive treatments include physiotherapy, orthotics and local steroid injections. Surgical treatments range from lumpectomy or wide local excision to subtotal or radical fasciectomy with or without skin grafting. Small surgical series (30 or fewer patients in each series) have reported recurrence rates of 30–40% and a significant chance of postoperative complications such as wound healing problems, chronic pain and poor functional outcome.
Radiotherapy
Radiotherapy seems to be an effective modality of treatment for plantar fibromatosis, with good local control and symptomatic benefit.
The planning target volume is usually palpable disease with a 2cm safety margin. The recommended total dose would be 30 Gy in ten fractions, given in two separate phases of 15 Gy in five daily fractions, with 12 weeks between the two phases. The radiotherapy can be delivered using orthovoltage photons or electrons as described above for Dupuytren’s radiotherapy. The risk of a radiation-induced skin cancer is likely to be 0.02% above background (24 ± 0.26%).
Salivary gland pleomorphic adenoma
Background
Pleomorphic adenomas are benign tumours of salivary glands, arising most commonly in the superficial lobe of the parotid gland. Other salivary glands are involved less frequently. Clinical presentation is typically with a painless slow-growing mass, which, if left untreated, can lead to significant morbidity. A sudden change in size suggests malignant transformation. Approximately 3–4% of pleomorphic adenomas can become carcinoma. Diagnosis is made on the basis of clinical history, imaging and a fine-needle aspirate.
Management
Multiple retrospective series report very high local control of >95% following surgical excision with clear margins. Therefore, surgery is the treatment of choice.
Radiotherapy
Adjuvant radiotherapy improves local control in subsets of patients and is recommended for patients who are at a higher risk of recurrence, as indicated by incompletely resected tumours, positive margins or multifocal recurrences.
For parotid pleomorphic adenomas the target volume includes the whole parotid bed.
Variable radiotherapy doses are reported in the literature with no clear evidence of dose response. Although higher doses similar to those used for malignant salivary disease have been used, doses in the region of 50 Gy in 25 fractions over five weeks have been commonly employed with good outcomes.
There is a small risk of long-term tissue damage in the radiation field with potential for developing radiation-induced cancers; this is less in older patients. It has been shown that both benign and malignant tumors can develop after radiation exposure, although the risk is very low with a latency of 6–32 years.
Sialorrhea
Background
Sialorrhea can be defined as excessive saliva in the mouth, resulting either from hypersecretion or facio-bulbar weakness. Patients can experience impaired swallow function, limited lip seal and saliva control, and consequently drooling. Drooling can be a feature of several neurological disorders such as amyotrophic lateral sclerosis, Parkinson’s disease, pseudobulbar palsy, stroke and cerebral palsy. Sialorrhea may increase risks of choking and aspiration. In addition, sialorrhea can have a major impact upon quality of life leading to social dysfunction, increased difficulty speaking, isolation and depression.
Management
Treatment for sialorrhea should be considered when quality of life is adversely affected. Anticholinergic medication is often utilised as a first-line pharmacological treatment and is effective in approximately 70% of patients with mild-to-moderate drooling. However, many patients experience significant side-effects and have to discontinue treatment. Botulinum toxin can be injected locally to reduce saliva production by reducing cholinergic parasympathetic and post-ganglionic sympathetic activity. Botulinum toxin is well tolerated, although requires frequent repeated injections. Several surgical procedures have been attempted, including salivary duct repositioning, denervation procedures and parotidectomy. These invasive procedures are mainly considered in younger patients resistant to medication and botulinum injections, and would rarely be considered in older patients or in patients with progressive neurological disorders and limited life expectancy.
Radiotherapy
Radiotherapy is an effective treatment modality in palliating sialorrhea in patients with advanced neurodegenerative disorders.
Recommended schedules include 20 Gy in four fractions over two weeks (two fractions per week) and 12 Gy in two fractions over one week. Retreatment may be more commonly required after the lower dose of 12 Gy in two fractions. Target volume usually includes both submandibular glands and caudal two-thirds of both parotid glands.
Data on retreatment is very limited but it can be effective.
The risk of radiation-induced cancer is very small since the dose is relatively low. However, in the rare cases where children might be considered for this approach, radiotherapy is not advised due to the potential risks of radiation-induced cancer and growth arrest leading to facial asymmetry. Other long-term toxicity is almost exclusively xerostomia/thick saliva.
Vestibular schwannoma (VS, acoustic neuroma)
Background
VS are benign tumours arising from the Schwann cells of the vestibular portion of the eighth cranial nerve. Patients classically present with asymmetrical hearing loss (>90%) and tinnitus with or without balance issues. Other symptoms include altered sensation or pain in a trigeminal distribution and for large lesions, facial nerve weakness or symptoms of brainstem compression or hydrocephalus (obstructive or communicating). Increasingly VS are diagnosed incidentally on scans for other indications. The majority of VS are ‘sporadic’ unilateral lesions. However, 4–6% of patients with VS have neurofibromatosis type 2 (NF2) and classically present with bilateral VS at a much younger age.
Management
Patients with sporadic vestibular schwannoma should be managed by an MDT with expertise in all therapeutic options. VS in the context of NF2 and schwannomatosis should be managed in conjunction with specialists in these genetic conditions as other options such as Bevacizumab may be considered. Initial management for all but the largest lesions should be active surveillance to assess rate of enlargement.
Surgery for VS is complex with a mortality of around 0.5% and acute complications such as cerebrospinal fluid (CSF) leak, hemorrhage, infection, facial nerve palsy and trigeminal nerve dysfunction. As a consequence, surgery should be considered for large VS compressing brainstem. Surgery should also be considered in those presenting at a young age where lifetime risk of late effects, including secondary malignancy, is greater.
Radiotherapy
Stereotactic radiosurgery (SRS) is now standard treatment for small but enlarging VS, with a marginal dose of 12–13 Gy in one fraction being the current standard.
Hypofractionated or conventionally fractionated RT can be considered for patents with large lesions when the patient is medically unfit for surgery or wishes to avoid surgery. Total doses ranging from 45 to 54 Gy given in 25–30 daily fractions of 1.8–2.0 Gy are currently recommended. Within this range, the fractionation of 50 Gy in 30 fractions over six weeks is very well tolerated.